15 July 2021
iSTAR Medical receives US FDA approval to start pivotal trial for MINIject in glaucoma patients
World-leading surgeons to join iSTAR Medical’s STAR-V study
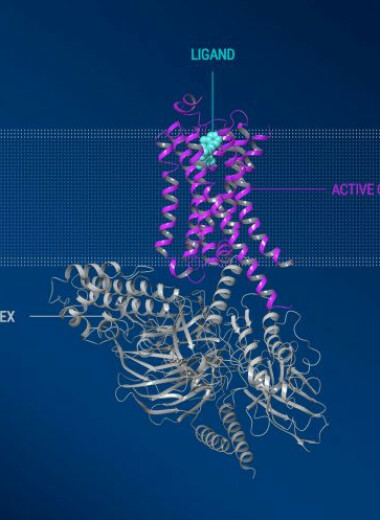
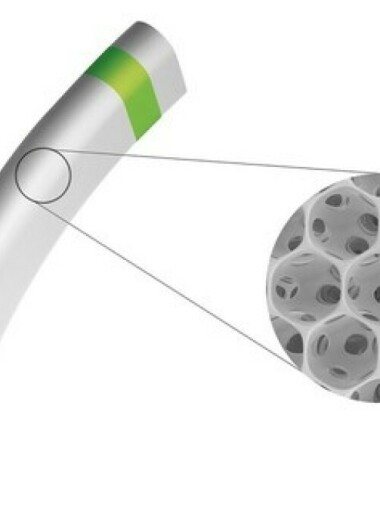
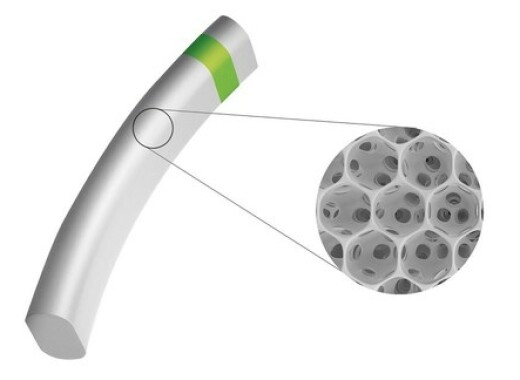
iSTAR Medical, a med-tech company developing minimally-invasive implants for glaucoma surgery (MIGS), announced today that the U.S. Food and Drug Administration (FDA) granted it Investigational Device Exemption (IDE) to start a pivotal trial with MINIjectTM. The STAR-V study will investigate MINIjectTM in over 350 patients with primary open angle glaucoma. World-leading glaucoma surgeons in the US, Canada and Europe will join the trial. The STAR-V trial evaluates MINIject’s efficacy by the mean reduction in eye pressure, as well as the proportion of patients achieving at least a 20 percent reduction in eye pressure. This study will report on safety and efficacy of MINIject alone, in a procedure not combined with simultaneous cataract surgery. Key study findings will become available when all patients have completed two years in the study. Patients will also be followed to evaluate long-term benefits and tolerability of MINIjectTM in the treatment of mild to moderate glaucoma.
More info on iSTAR Medical‘s website.